Sulphuric Acid Strong or Weak
Hydrochloric acid and sulphuric acid are examples of. Set the apparatus as shown in the Fig.

Is H2so4 An Acid Or Base Strong Vs Weak Sulfuric Acid
COO react with dilute sulphuric acid to evolve different gases.

. A strong acid is the one that completely ionizes in a solution whereas a weak acid only partially ionizes. For example to prepare 500 cm 3 of 015 mol dm-3 sulphuric acid from a stock solution of 20 mol dm-3 sulphuric acid. Of the common strong acids it is one of the least hazardous to handle least expensive and easiest to store.
A Calculate the volume of stock solution required. Here are some of the values of weak and strong acids and bases dissociation constants used by BATE when calculating pH of the solution and concetrations of all ions present. Sulfuric acid is a mineral acid with the chemical formula H 2 SO 4.
Hastelloy Alloy B-2 Cabot Stellite Division Brochure 1977. Calcium bi-carbonate is produced C. As well the formation of weak acid in the system may lead to increased corrosion and eventual leaks.
Study of the characteristics of the gases evolved gives information about the anions. The concern is that if water remains in the lines when sulphuric acid is introduced there may be a violent reaction due to the sudden mixing of water and acid. This time you get an equilibrium.
While colorless in pure form nitric acid yellows over time as it decomposes into nitrogen oxides and water. It is also known as phosphoricV acid or orthophosphoric acid. 7 to 10 B.
Alloy piping system are more easily damaged by weak acid than ductile iron systems. Sulphuric acid carefully along the sides of the test tube. Run out 375 cm 3 of stock solution into a 500 cm 3 volumetric flask containing some distilled water.
7 to 14. It is also used in making fertilizers cleaning products and manufacturing of polymers. In fact the hydrogensulphate ion is a relatively weak acid - similar in strength to the acids we have already discussed on this page.
Nitric acid also goes by the name aqua fortis. Phosphoric acid is a strong acid. These are picked up by the ethanoate ions or propanoate ions or whatever present.
In chemistry one of its key uses. It dissociates in an aqueous solution and produces hydronium H PO 4 ions. Range of pH scale is A.
It is a highly corrosive acid. Due to excess passing of CO2 through an aqueous solution of slaked lime its milkiness fades because A. Students can get a complete understanding of this topic on the official site of Vedantu where the experts have provided.
A separate Monograph on Mists from Strong. Due to the production of. Procedure a Take 01 g of the salt in a test tube and add 12 mL of dilute sulphuric acid.
Sulphuric acid H₂SO₄ B. Summary of characteristic properties of gases is given in Table 71 below. In organic chemistry and biochemistry important examples include amino acids and derivatives of citric acid.
In lead-acid batteries the concentration of sulfuric acid in water ranges from 29 to 32 or between 42 molL and 50 molL. As a result the aqueous solution is pH neutral. Iron and steel industries use sulfuric acid.
Isocorrosion Curves Sulphuric Acid Sulphuric Acid 2000 ppm Chlorides. Battery acid is highly corrosive and able to cause severe burns. I was looking mainly for density table of citric acid solutions now I am impressed with the ease of calculations using CASC.
Phosphoric Acid is an acid-containing four atoms of oxygen one atom of phosphorus and three atoms of hydrogen. If you do this the mixture is flooded with hydrogen ions. Calcium oxide is produced D.
Since that time new data have become available which have been incorporated in this Monograph and taken into consideration in the present evaluation. In terms of supply the. When sodium carbonate is dissolved in water it is partially hydrolyzed yielding sodium hydroxide and carbonic acid.
Other examples of inorganic polyprotic acids include anions of sulphuric acid phosphoric acid EDTA and hydrogen sulphide that have lost one or more protons. Sodium hydroxide is now a strong base that is fully ionised and. Sulphuric Acid Sulphuric Acid 200 ppm Chlorides.
The most common application is the Sulphuric acid found in the car batteries. SHANGHAI Apr 27 SMM - SMM Morning Meeting. Corrosion Rates Reagent Grade Sulphuric Acid at 93C - Corrosion.
Orthophosphoric acid refers to phosphoric acid. Calcium carbonate is produced B. But the weak acids are only mildly corrosive and are even present in our food and body.
0 to 14 D. Sulphuric Acid Sulphuric acid is a strong acid whose single drop can cause a hollow mark in your skin. Bring a burning match stick near the mouth of the test tube Fig.
The value of pH for a weak acid is less than 7 and not neutral 7. Battery acid is a common name for sulfuric acid US or sulphuric acid UK. Aqueous sodium sulphate solution is hydrolyzed to create sodium hydroxide and sulphuric acid both of which are strong bases and acids.
If you want the acid rather than its salt all you have to do is to add an excess of a strong acid like dilute hydrochloric acid or dilute sulphuric acid to the solution left after the first distillation. Nonetheless there can be some exceptions as Hydrofluoric acids p H is 327 which is also low as strong acid hydrochloric acid with a pH value of 301. Isopropyl alcohol and isopropyl alcohol manufacture strong-acid process were considered by previous IARC Working Groups in 1977 and 1987 IARC 1977 1987.
Differentiate between strong and weak acids and bases. The salt calcium phosphate and water are the results of the reaction. Apart from its too dangerous property it has numerous applications.
Its pH value is less than that of strong acids. B Fill a burette with the stock solution. 0 to 10 C.
Stand Dilute Sulphuric acid Zinc granules Hydrogen gas bubbles Burning of hydrogen. Sulphuric acid and strontium hydroxide. The second hydrogen is more difficult to remove.
HSO 4-aq H 2 Ol H 3 O aq SO 4 2-aq Sulphuric acid of course has all the reactions of a strong acid that you are. Acid Rain Reading Comprehension Mosses Reading Comprehension Sinkholes Reading Comprehension Pesticides and Herbicides Reading Comprehension Traits Reading Comprehension The Five Kingdoms Reading Comprehension Heat Energy Reading Comprehension Scientific Names Reading Comprehension Static Electricity Reading. Strong acids are corrosive in nature and cause severe burns when they come in contact with skin.
Hastelloy Alloy C-276 Haynes International Bulletin H-2002B. Although an amphiprotic species must be amphoteric the converse is not true. Phosphoric Acid is a weak acid with the chemical formula H 3 PO 4.
It is present in teeth and bones and helps in. Explain the role of water in dissociation of acids and bases. Calcium hydroxide acts as a base dissociates in an aqueous solution to form Ca and OH-.
Observe the change. SHFE nickel remained high and spot transactions improved slightly but the overall supply and demand remained weak Although macro negative factors still existed SHFE nickel maintained a high as the shortage at the fundamental supply side still gave strong support to the nickel prices.

Is H2so4 An Acid Or Base Strong Vs Weak Sulfuric Acid
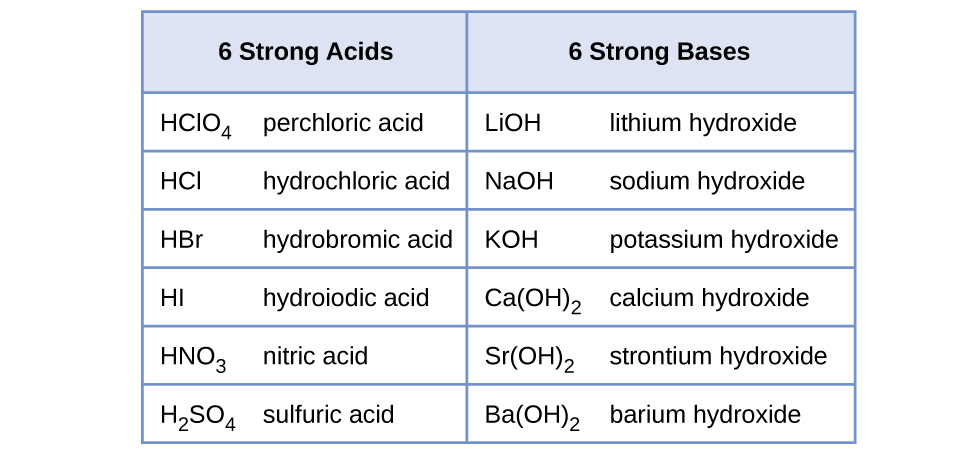
14 3 Relative Strengths Of Acids And Bases Chemistry 112 Chapters 12 17 Of Openstax General Chemistry

No comments for "Sulphuric Acid Strong or Weak"
Post a Comment